Benefits of Using Scarletred®Vision for Phase IV Post-Marketing Studies

Post-Marketing Studies play an integral role in gathering information about safety and efficacy on a large scale. However, monitoring adverse events and patient-reported outcomes in a real-world setting can be challenging.
After a drug or medical device has been approved, phase IV post-marketing studies are beneficial to monitor safety and long-term adverse events in a real-world setting after market release. This allows collecting data from a large number of patients with a variety of medical conditions. As in the earlier clinical study phases, data collection must be efficient, secure, and safe for the patients. The data is usually collected from various health databases, electronic health records, and patient registries. Yet, many post-marketing studies lack a systematic and controlled investigation with a centralized, long-term approach regarding safety and adverse events. To gather high-quality real-world data, long-term studies need to be highly organized, and data must be easily accessible for post-marketing analysis. This can be especially valuable for non-interventional studies.

Scarletred®Vision facilitates the collection of this valuable data by providing a single platform to compile the post-marketing information given by physicians and the patients themselves. Physicians can take photos, for instance, of drug-related side effects and the progress of treatment in various skin diseases and injection site reactions (ISR). The only required tool is the Scarletred® skin patch and the app on their smartphone. Another critical aspect besides product-related side effects is evaluating the patients’ quality of life. This is possible by implementing standardized and validated questionnaires documenting electronic patient-reported outcomes (ePROs), which can be accessed digitally or provided during your next check-up. With Scarletred®Vision, individual data is collected using a unique QR code, which links the patient images and ePROs for valuable real-world data. Honoring the strict General Data Protection Regulations, the QR code ensures that patients stay anonymous and their medical data is stored safely. This application for post-marketing studies is a highly efficient approach and can easily be implemented on a large scale to provide high-quality data.
How does Scarletred®Vision work?
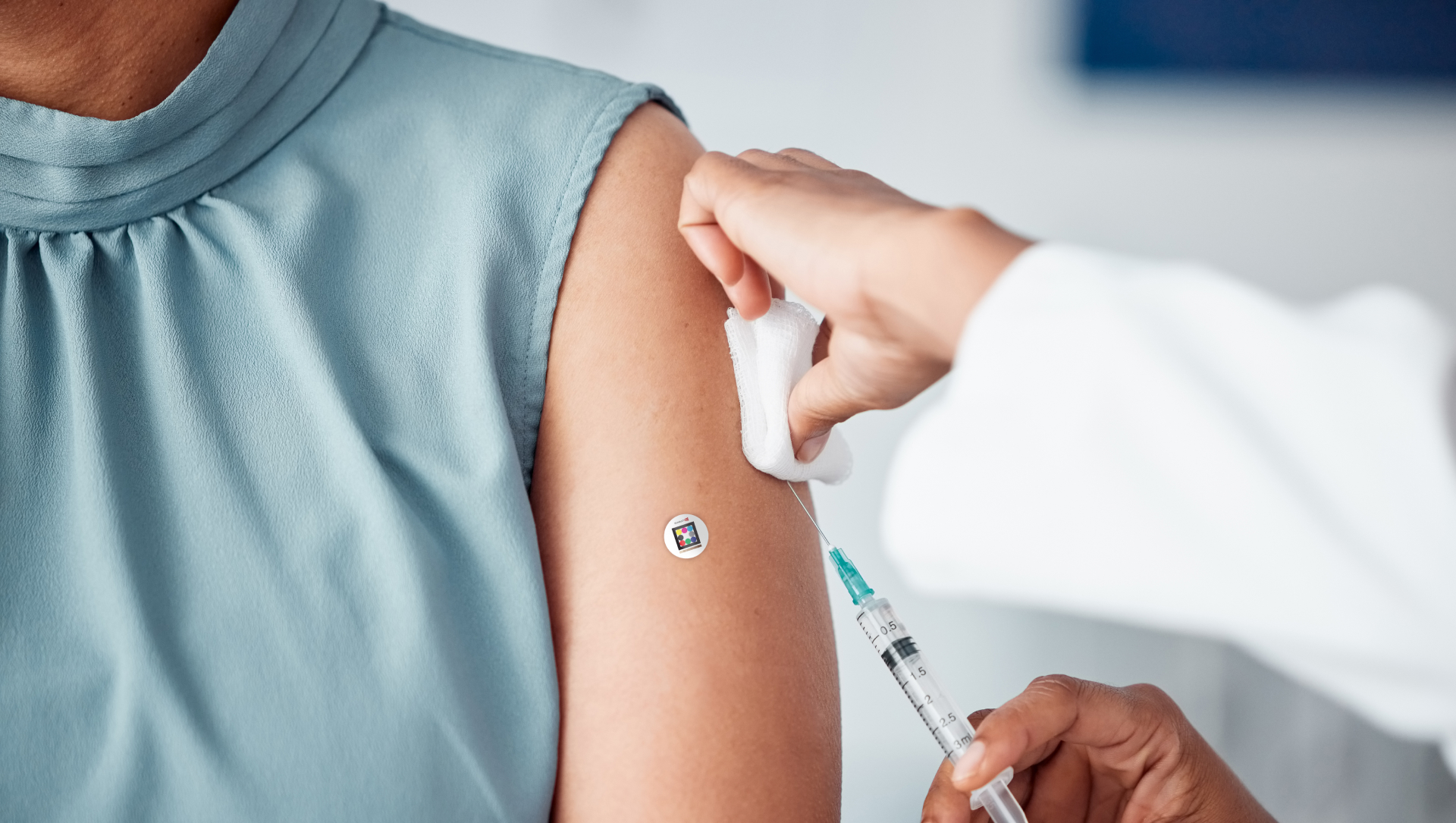
Scarletred®Vision is composed of 3 components:
- The mobile app, which allows the generation of high-quality anonymized images, ensuring patient privacy
- The specially designed skin patch, which allows the software to calibrate the image for light conditions and size/distance
- The online platform: a secure web-based platform that enables doctors and experts to document, assess, and quantify the affected areas
The imaging and analysis procedure consists of 3 steps:
- Place the skin patch on the healthy skin next to the area of interest
- Take an image using the mobile app (the image is then automatically uploaded to the secure online platform)
- The analysis can be performed on the online platform, which offers multiple tools to assist the physicians in their assessment
The technology displays the image in 4 different signal maps, each one highlighting a specific parameter: Standard Erythema Value (SEV*), redness (a*), yellowness (b*), and lightness (L*). Another essential parameter for rosacea assessment is the measurement of skin texture changes. To this purpose, Scarletred®Vision delivers a state-of-the-art tool to quantitatively assess these alterations.



To get your personalized offer, please reach out directly to us via office@scarletred.com or our contact form.